Stainless steel is no longer unfamiliar in daily life; it appears in most applications ranging from commercial to residential use. From kitchen equipment to structural stainless steel components It is widely chosen for its corrosion resistance, hygienic properties, and high aesthetic value. However, not all stainless steel has absolute corrosion resistance. However, many people are surprised to learn that stainless steel rust can still occur under certain conditions. So, what causes stainless steel to rust? The article below provides the main reasons why rust occurs and effective solutions to prevent it.
WHAT IS STAINLESS STEEL?
Characteristics of Stainless Steel
Stainless steel is the general name for a group of corrosion-resistant alloy steels that contain a minimum of 10.5% chromium. This chromium layer forms an extremely thin protective oxide film on the surface of the steel to resist corrosion that causes rust.
In addition to chromium, this group of alloy steels also contains elements such as nickel, molybdenum, titanium, niobium, and other elements.
The Origin of Stainless Steel
Stainless steel was actually discovered by accident by British metallurgist Harry Brearley in 1912. Initially, he was researching an alloy made of iron, chromium, carbon, and nickel to prevent corrosion in gun barrels. However, the alloy did not meet the original requirement and was left outdoors for a long time. Later, Harry Brearley noticed that it had not rusted and remained shiny. After this accidental discovery, stainless steel was developed and has been widely used up to today.
Stainless Steel Grades
- 303 Stainless Steel
- 304/304L Stainless Steel
- 316/316L Stainless Steel
- 201 Stainless Steel
- 430 Stainless Steel
TOP REASONS FOR STAINLESS STEEL RUST
-
Chloride-Rich Environments
Chloride-rich environments are the leading enemy of stainless steel. Even high-quality stainless steels such as 304 or 316 can corrode and rust in these conditions. Chlorides are abundant in seawater, human sweat, and chlorine-containing cleaning agents such as those used in swimming pools.
To prevent stainless steel from rusting in these specific environments, 304 or 316 stainless steel should be prioritized, and an additional protective surface coating should be applied to prevent direct exposure to chloride-rich environments.
-
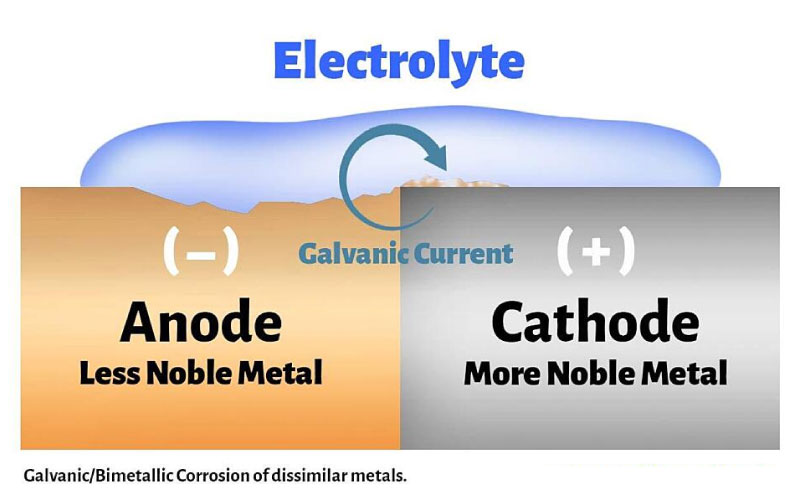
Galvanic/Electrochemical Corrosion When Welding Different Stainless Steel Alloys
Some inexperienced stainless steel fabricators weld or join different types of stainless steel together. Because each grade has different properties, joining them can create electrochemical reactions, causing the metal that more easily loses electrons to corrode faster.
Examples:
Welding 304 to 316 makes 304 corrode faster
Welding 316 to Duplex 2205 makes 316 corrode faster
Welding Austenitic to Ferritic (430) makes 430 corrode faster
Welding stainless steel to carbon steel makes the carbon steel corrode faster
To avoid this type of stainless steel rust, suitable filler materials should be selected, and different grades should not be welded together arbitrarily.
-
Metal Dust Contamination
In some cases, dirt and dust from machining carbon steel or iron can adhere to the surface of stainless steel. These particles tend to rust first, and the rust stains spread, making the stainless steel appear to be rusting.
To prevent this type of corrosion, dedicated stainless steel fabrication areas should be arranged and thoroughly cleaned before working with stainless steel.
-
Excessive Heat During Welding
Although stainless steel has a relatively high melting temperature, this does not mean it can resist corrosion at high temperatures. Excessive heat during stainless steel fabrication, especially welding, can form oxide scale on the metal surface or even burn off the protective chromium layer. This creates weak points where rust and corrosion can begin.
To prevent this type of stainless steel rust, moderate welding current should be used and the time the metal stays in the temperature range of 450–850°C should be minimized.
-
Lack of Oxygen
The passive film, only a few nanometers thick, acts as a protective shield that helps stainless steel resist rust. It mainly consists of chromium oxide and has the ability to self-repair when oxygen is present. If the surface is scratched but oxygen is available, this film can still restore itself. In contrast, a lack of oxygen disrupts this mechanism and accelerates stainless steel rust, especially in chloride-rich environments.
Common causes of oxygen deficiency on stainless steel surfaces include prolonged water immersion, coverage by grease or dirt, or installation in tight, unventilated crevices.
To minimize this issue, designs should avoid narrow gaps, ensure proper drainage and ventilation, prevent stagnant water, and perform periodic cleaning to remove deposits.
It can be seen that most causes of stainless steel rust mainly come from improper fabrication or installation. If you are not highly experienced with stainless steel, it is best to contact reputable and experienced stainless steel fabricators to ensure optimal performance for your project.
If you need a high-quality stainless steel fabricator offering tailored metal solutions that meet international standards.
Contact Newinds:
Email: sales@newindscorp.com
Phone/Whatsapp/Zalo: Ann Yen +84 868 482 038